Abstract
Ischemic injuries and subsequent degenerative myocardial and conduction system abnormalities occur in patients with sickle cell disease (SCD). This may lead to conduction and repolarization delays reflected as QTc prolongation. Patients with SCD have increased risk of cardiac death, the basis of which remains uncertain. QTc prolongation may be a contributing factor. We decided to examine factors that can potentiate QTc prolongation in this population. In particular, we studied effect of iron overload estimated by serum Ferritin level on QTc.
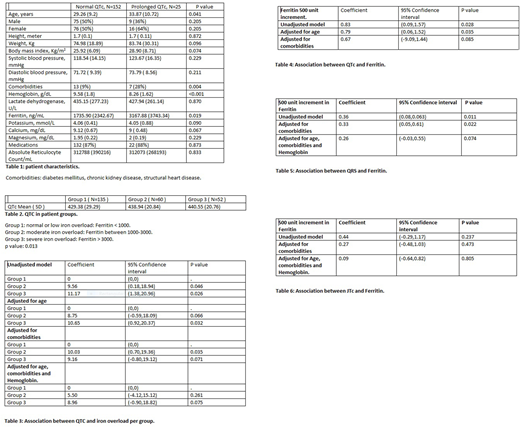
We performed a cross-sectional study in SCD patients older than 18 years, in steady state, followed in our clinic. Patients with acute illness or vaso-occlusive crisis in prior 2 weeks, patients with bundle branch block, pacemaker or arrhythmia and patients unable to give consent were excluded. Prolonged QTC was defined as >450 and >460 ms in men and women respectively. Patients were divided into 3 groups corresponding to mild, moderate and severe iron overload with Ferritin < 1000, between 1000 and 3000 and > 3000 ng/mL respectively.
QTc was prolonged in 25/177 patients (14%). Those were older (p=0.041), had lower hemoglobin (Hb) (p<0.001) and higher Ferritin (p=0.019). Their mean age was 33.8 years, Hb 8.26 g/dL and Ferritin 3167 ng/mL compared to 29.2, 9.58 and 1735 respectively in patients with normal QTc. Twenty eight % of patients with prolonged QTc had comorbidities compared to 9% of patients with normal QTc (p=0.004). There was no difference between the 2 groups regarding gender, weight, blood pressure, Lactate dehydrogenase, electrolytes, Reticulocytes count or use of medications known to prolong QTc. Mean QTC was 429, 438 and 440 ms in groups 1,2 and 3 respectively (p=0.013). Linear regression analysis showed that QTc is expected to be longer in groups 2 and 3 compared to group 1. We also estimated QTc prolongation corresponding to 500 unit increment in Ferritin. QTc is expected to get prolonged by 0.83 ms for each 500 unit increment of Ferritin with p value of 0.028 in unadjusted model and by 0.79 ms with p value of 0.035 and 0.67 ms with p value of 0.085 in models where age and comorbidities were adjusted for respectively. Hb was found to be inversely correlated with Ferritin. Correlation coefficient was -0.39 with p value < 0.001. Although there was no significant correlation between Ferritin and JTc, analysis showed that QRS is expected to increase by 0.33 ms for each 500 unit increment in Ferritin with p value of 0.022.
Comorbidities including diabetes mellitus, kidney and heart disease are known independent factors that can cause QTC prolongation. QTc increases with age. However, this mainly applies to people older than 50 years (J Geriatric Cardiol 2016 Sep;13(9):740-8). We don't believe age is an independent QTc prolonging factor in our patients especially that mean age of patients with prolonged QTc was 33.87 years. Probably older patients had longer exposure to iron toxicity which may be the true contributing factor to QTc prolongation. Patients with prolonged QTc had lower Hb. However, no correlation was found between Hb and QTc in patients with anemia caused by conditions other than SCD (Chin Med J 2015 Dec 20;128(24):3385-6). In addition, major cause of tissue injury in SCD patients is intracellular polymerization of HbS. However, there is no correlation between Hb concentration and intracellular HbS polymer content (Blood 1998 Mar 1;91(5):1777-83). Thus, we don't think Hb is an independent QTc prolonging factor. Probably patients with lower Hb received more transfusions and subsequently had more pronounced iron overload which may be the direct contributing factor to QTc prolongation. The negative correlation between Hb and Ferritin supports our hypothesis. Thus, we think that the model where just comorbidities were adjusted for is the best to reflect the association between Ferritin and QTc.
Iron overload reduces overshoot ( Circulation 1999 Aug 10;100(6):675-83) which will compromise propagation of cardiac impulse and result in conduction delay. Iron also leads to production of free radicals. That will cause chronic inflammation and fibrosis. We showed that QRS is expected to get prolonged with iron overload in SCD patients which is consistent with the physiology of iron toxicity.
QTc prolongation seems to be associated with iron overload in SCD patients. Conduction delay manifested by prolonged QRS may be the main contributor rather than repolarization delay.
Bader:NIMHD: Research Funding. Majumdar:NIMHD: Research Funding. Maher:NIMHD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal